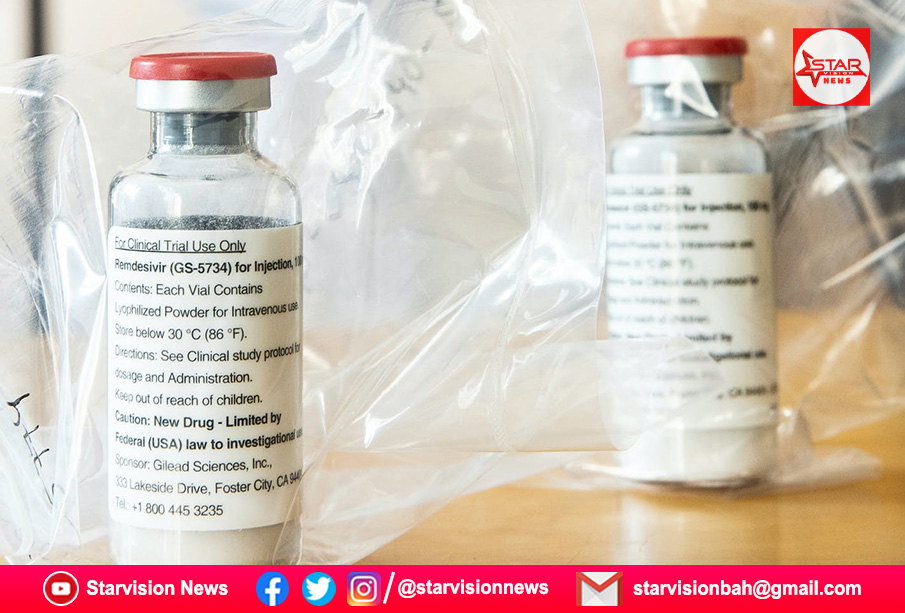
India’s government said on Tuesday it has approved Gilead Sciences Inc’s antiviral drug remdesivir for emergency use in treating COVID-19 patients.
Remdesivir is the first drug to show improvement in COVID-19 patients in formal clinical trials. It was granted emergency use authorization by the U.S. Food and Drug Administration last month and has received approval by Japanese health regulators.
The drugmaker did not immediately respond to an email seeking further details.
Gilead Sciences had on Monday reported that remdesivir showed modest benefit in patients with moderate COVID-19 given a five-day course, while those who received it for 10 days in the study did not fare as well.
European and South Korean authorities are also looking at remdesivir, with South Korean health authorities last Friday saying they would request imports of the drug. Gilead is yet to gain regulatory approval in either market.
Also Read:
Movement permits required in Abu Dhabi from tomorrow
George Floyd’s died from asphyxia, private autopsy says
‘I can’t breathe’ protest raise fears of new virus outbreaks
No evidence potency of coronavirus changing: WHO